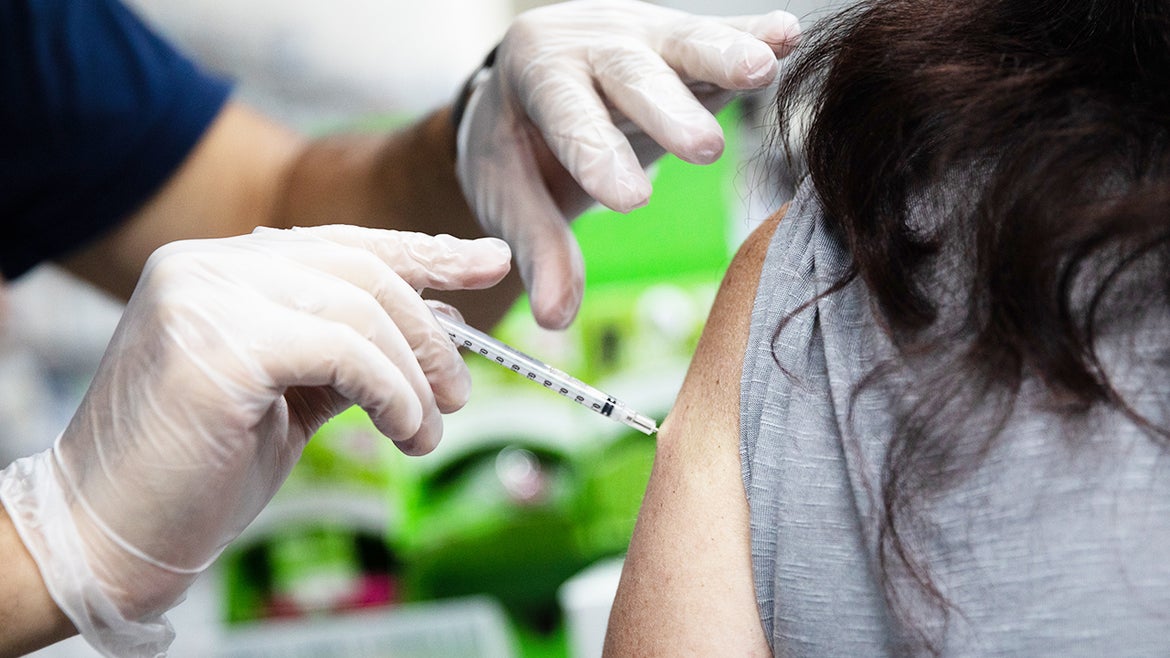
The Centers for Disease Control (CDC) has expanded booster shot eligibility to encompass as many as 99 million Americans, including those who live in correctional facilities or have mental health conditions.
More and more fully vaccinated Americans are now eligible for a COVID-19 booster shot as the Centers for Disease Control (CDC) signs off on sweeping recommendations for those who already received two shots of Moderna or Pfizer, or one shot of Johnson & Johnson, and discussion begins around a possible fourth dose.
And as the new recommendations, which have also received Food and Drug Administration (FDA) approval, now encompass those with mental health conditions, up to 99 million Americans are now eligible to receive a booster shot, according to CBS News.
FDA Commissioner Janet Woodcock, M.D. explained the expanded guidelines are meant to support those most vulnerable. "The available data suggest waning immunity in some populations who are fully vaccinated. The availability of these authorized boosters is important for continued protection against COVID-19 disease," she said in a statement.
Inside Edition Digital spoke to Dr. Robert G. Lahita, Director of the Institute for Autoimmune and Rheumatic Disease at Saint Joseph Health, also known as Dr. Bob, about the latest decision.
“These people will benefit from the booster shot with these variants popping up in increased frequency,” said Lahita, author of the newly released book, "Immunity Strong."
Here’s what we know so far.
Who’s Eligible for the COVID-19 Booster Shot?
Those who received the Johnson & Johnson's Janssen COVID-19 shot at least two months ago and are at least 18 years old can receive another dose of the Johnson & Johnson vaccine.
Those who received the Moderna or Pfizer vaccine shot can receive a booster shot six months after the initial vaccination if they are over 65 years old, or are over 18 years old and live in long-term care settings, have underlying health conditions, or live or work in high-risk settings.
According to the CDC, long-term care settings include inpatient psychiatric or rehabilitation facilities, and high-risk settings include correctional facilities and homeless shelters. A complete list can be found here.
The CDC also expanded their criteria for underlying health conditions, which was once mostly comprised of physical conditions, to include mental health conditions, including depression, bipolar disorder and schizophrenia spectrum disorders. A complete list of eligible health conditions can be found here.
If you want to know whether your condition with increased risk qualifies for a booster shot, speak to your doctor. Those with a history of myocarditis or pericarditis should consult their doctor before receiving a booster shot.
What Does It Mean to Mix-and-Match Doses?
Vaccinated people who originally received Pfizer or Moderna vaccine were given a second dose of the same vaccine, the CDC is now allowing those eligible, including those who received Johnson & Johnson, to choose which vaccine they prefer for their third dose.
Mixing-and-matching is safe, according to health authorities, and some may make a decision based on how their body reacted to the initial vaccination.
Those who have received the Johnson & Johnson vaccine, a vector-based vaccine, in particular may want to mix-and-match with Pfizer or Moderna, mRNA vaccines, as each type of vaccine triggers different responses in the immune system that helps fight off COVID-19, according to Science.org.
"Two different vaccines may be more potent than either vaccine alone," said Harvard professor Dan Barouch, who helped develop the Johnson & Johnson vaccine, according to Science.org.
Mixing-and-matching doses also helps make the best use out of vaccine availability, and many countries like Canada that had once faced vaccine shortages have already been safely administering different doses of vaccines.
The CDC and FDA did not made official recommendations any specific combination of shots individuals should receive.
How Do COVID-19 Booster Shots Work?
A shot of any vaccine triggers your immune system to recognize the vaccine as the disease, thereby activating your body to produce antibodies to fight against it, according to the World Health Organization (WHO). The additional shot of the COVID-19 vaccine will reactivate the body to fight against the coronavirus, training your body over time to recognize and better defend itself against the coronavirus should you be exposed.
“These boosters ‘boost’ the immune systems' responses to seeing the COVID virus so if someone gets sick, it will not be a very severe infection,” Lahita said.
Have The Booster Shot Guidelines Changed?
The original FDA approval came in August, and allowed only certain immunocompromised people to receive a booster shot as many states across America saw surges of positive cases and hospitalizations during a spike of the highly contagious Delta variant.
"The FDA is especially cognizant that immunocompromised people are particularly at risk for severe disease," Woodcock said in an earlier statement. “[The] action allows doctors to boost immunity in certain immunocompromised individuals who need extra protection from COVID-19."
The earlier guidelines only recommended booster shots to those who have received a solid organ transplant or those with a similar level of immunocompromise. According to Lahita, examples of eligible conditions include lupus, rheumatoid arthritis, leukemia, lymphoma, vasculitis and HIV.
“It's important for these groups to get the extra shot because their immune systems are weakened because of their conditions, and thus they are more susceptible to getting COVID,” Lahita said. “They should not hold off."
When Can 11-Year-Olds and Younger Get Vaccinated?
While COVID-19 vaccines are currently only eligible to those 12 years old and older, vaccine access for those 5 to 11 years old is expected in the upcoming days.
A vaccine committee advising the FDA voted unanimously to emergency use authorization of the Pfizer vaccine on children aged 5 to 11. If approved, the younger children will be given a one-third dose of the vaccine in hopes it will cause fewer side effects, according to CNN.
What About Americans Who Are Still Unvaccinated?
More Americans are now receiving booster shots than those receiving initial doses, according to CDC data analyzed by the Washington Post, with more than two people receiving a booster shot to each person receiving an initial dose.
"There is a lot of vaccine hesitancy," Lahita said, emphasizing that it is important to remind unvaccinated friends and family the vaccine is safe and effective.
While there continues to be controversy surrounding vaccine public health mandates across the country, the mandates have proven to be effective, with many companies and industries seeing a significant increase of vaccinated staff ahead of deadlines.
Will There Be a Fourth Dose?
While most people will only require a third shot of the vaccine, the CDC is recommending certain "moderately to severely immunocompromised" people, which makes up about 3% of the population, to receive as many as four doses of the COVID-19 vaccine to support their a weakened immune response.
Those eligible for a fourth dose include individuals receiving cancer treatment, have received an organ transplant or have either untreated or advanced stage HIV. A complete list can be found here.
The guidelines to receiving the third shot still applies, and for those who fall into the category that requires a fourth shot will wait at least 28 days after receiving either the Moderna or Pfizer booster shot before receiving their fourth shot.
What About the WHO’s Plea for a Moratorium on COVID-19 Booster Shots?
Over the summer, the World Health Organization (WHO) called for a booster shot moratorium, asking developed nations to stop distributing additional vaccines until the goal of vaccinating 10% of the population of each country is reached.
“We need an urgent reversal from the majority of vaccines going to high-income countries, to the majority going to low-income countries,” WHO Director General Tedros Adhanom Ghebreyesus said in a statement.
Their goal is to have 40% of the world vaccinated by December, and 70% of the world vaccinated by the middle of 2022, according to CNBC.
As of the summer, 10 countries have administered more than 75% of the world’s vaccines, with developing countries receiving just 1% of the world’s vaccines, Time reported.
There are at least 10 countries that have vaccinated less than 1% of their populations, according to research by The New York Times.
“It’s a disaster,” Lahita said. “Why are we starting with third shots when we have so many who don't even have their first shots yet?”
He added that reaching the WHO’s goal is crucial to reaching worldwide heard immunity, thereby effectively eliminating the COVID-19 and ending the pandemic.
“But I don't see us getting there anytime soon,” Lahita said. "If we could immunize most of the population, we wouldn't have a problem.
However, those who qualify for a booster shot in the United States as per the FDA’s new guidelines should not wait to get vaccinated, Lahita urged.
“There is not really anything the individual can do to support those in developing countries and their vaccination goals – they don't have the power,” he said. “All they can do is encourage and convince their family, friends and neighbors to get vaccinated.”
If You Don’t Fall in The Categories Mentioned, Should You Get a Third COVID-19 Vaccine Dose?
Not yet.
According to the FDA, “other individuals who are fully vaccinated are adequately protected and do not need an additional dose of COVID-19 vaccine at this time.”
However, they clarified that rules and requirements are continuing to change as new research and data comes out. “The FDA is actively engaged in a science-based, rigorous process with our federal partners to consider whether an additional dose may be needed in the future,” the statement read.